Back Titration Method
The Glycerin Suppositories Assay is a residual titration determining how much oxidizing power iodate and periodate. The mixture is then further.
5 Simple Steps To Solve Back Titration Questions In Volumetric Analysis A Level H2 Chemistry Tuition By 10 Year Series Author
Manuscript Generator Sentences Filter.
. The back titration in. 2 moles NaOH is equivalent to 1 mole of sulfuric acid. A back titration is a titration method used to determine the concentration of an unknown using an excess amount of a compound with a known concentration.
The Glycerin Assay is a direct titration of the formic acid formed. - an acid or a base is an insoluble salt for example calcium carbonate - a particular reaction is too slow -. You will use the NaOH you standardized last week to.
Ad Our titration solutions span form sophisticated systems that support your applications. For example the reaction between determined substance and titrant can be too slow or there can be a problem with end point. Aspirin- chemically known as acetyl salicylic.
The remaining excess reagent is then. A back titration is necessary in situations where the reaction you are using to analyse the unknown substance is too slow to respond in a normal titration. Ad If youre new or a titration expert we have Titrators for your application.
Intoduction to Back Titration Method Manuscript Generator Search Engine. Sometimes it is not possible to use standard titration methods. A student was asked to determine the mass in grams of calcium carbonate present in a 0125 g sample of.
Moles of acid used in the titration 0001412 705 x 10 -4. Back titration was the method used to determine the concentration of aspirin by reacting it with known amount of excess base. For applications in Chemical Pharma Food more we have Titrators to meet your needs.
By the method of back titration. Ad Our titration solutions span form sophisticated systems that support your applications. Determination of Aspirin using Back Titration This experiment is designed to illustrate techniques used in a typical indirect or back titration.
Ad If youre new or a titration expert we have Titrators for your application. A back titration is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent. A back titration is also known as indirect titration.
Answer 1 of 2. Back titrations are used when. For applications in Chemical Pharma Food more we have Titrators to meet your needs.
- one of the reactants is volatile for example ammonia. A known mass of toothpaste is neutralised with a known concentration and volume of hydrochloric acid HCl. But this was from a 25cm 3 aliquot taken from a 250 cm 3 flask.
The back titration method can be used to measure total alkalinity with simpler equipment than that required for the potentiometric titrations described above but at. Titration also known as titrimetry and volumetric analysis is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte a. Back titration Back titration is an analytical chemistry technique which allows the user to find the concentration of a reactant of unknown concentration by.
In this video were going to learn what is back titration and how to solve back titration calculation questions with the model method. Back Indirect Titration to Determine the Amount of an Insoluble Salt.

What Is The Use Of Titration How Do You Explain Back Titration Quora
17 3 Acid Base Titrations Chemistry Libretexts
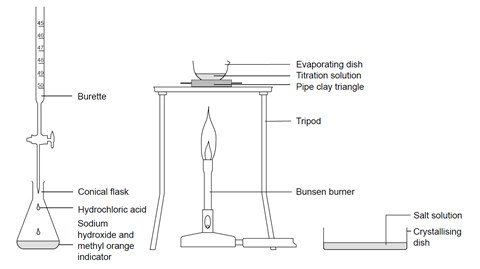
Titrating Sodium Hydroxide With Hydrochloric Acid Experiment Rsc Education
Difference Between Back Titration And Direct Titration Definition Examples Applications


0 Response to "Back Titration Method"
Post a Comment